Can Biocon Join The $1-billion Club By 2018?
Kiran Mazumdar-Shaw wants the biotech company to break into the $1-billion club by 2018. But with stiff competition, restrictions on drug trials and political uncertainty in the Middle East, it will n


Kiran Mazumdar-Shaw is on a tight deadline. Plans she had set in motion at least a decade ago are nearing fruition. According to the timetable, the founder chairman and managing director of Biocon has four years to increase the biopharmaceutical company’s revenues from its current $487 million to $1 billion. This is an ambitious goal but, then, Mazumdar-Shaw (61) is yet to shy away from a challenge. “Risks, after all, are part of an entrepreneur’s life,” says the businesswoman who started the company in a garage in 1978.
In 36 years, Biocon has evolved from an enzyme-manufacturing company to a complex drug discovery and production business. It is the largest biologics company in India (a biological drug is made from a variety of natural sources such as humans, animals or microorganisms), and the fourth largest insulin provider in the world. Its drug discovery pipeline straddles the worlds of cancer and diabetes, two of the five most profitable diseases for big pharma companies.
With the launch of its novel biological psoriasis drug Alzumab in 2013 and its biosimilar breast cancer drug early this year, Biocon has made it clear that it is a determined and hungry player in the competitive pharmaceutical industry. (A biosimilar product is “highly similar” to a pioneer drug and is a follow-on version of original biological medicines.)
And it does not want to lose its momentum. Biocon is getting ready to take its subsidiary company Syngene, a contract research and manufacturing arm, public within the next few months.
A lot, however, could go wrong. Its multi-million dollar insulin production facility in Malaysia will be commissioned in 2016, but its operational status is dependent on clearances from local regulators. Its oral insulin drug, which is touted to be a game changer in managing diabetes, is still under trial. It could take at least another two years to hit the market. If the trials are successful, then Biocon’s future success is assured. If not? Mazumdar-Shaw refuses to entertain that thought. “Getting into novel programmes was a risk. I understand risks well and manage them in a way that it doesn’t impact the company badly,” she says. She has, however, hedged her bets: Biocon’s $1 billion target is not dependent on either the drug or the Malaysian plant.
Meeta Shetty, an analyst with HDFC Securities who tracks the company regularly, says riding risks is not unusual for companies such as Biocon and Dr Reddy’s Laboratories. They not only have research and development (R&D) units but have also established lines of business in generics and branded formulations. “These companies are not just waiting for one under-discovery drug or molecule to click. They have a well-balanced approach. It’s a balanced risk… they do research and keep making profits,” she says.
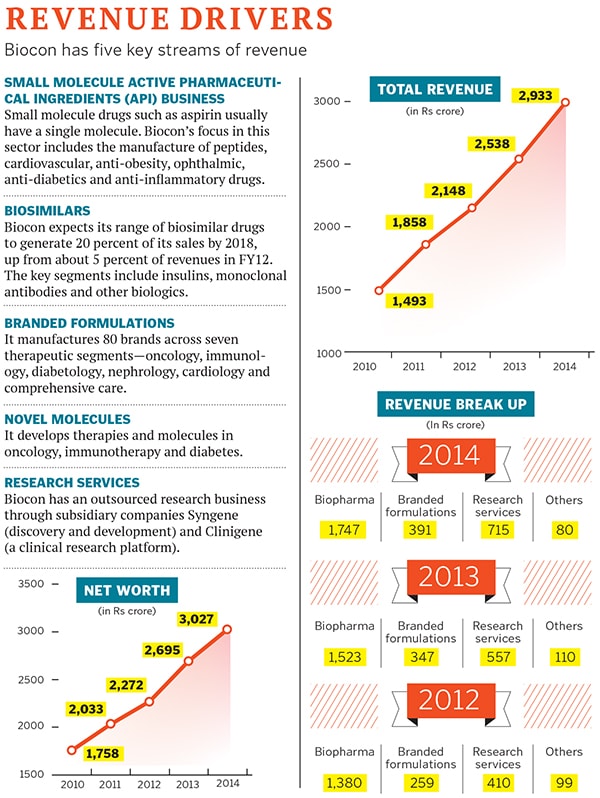
Biocon follows a similar model. Apart from its research services, Syngene and Clinigene (a clinical research organisation), the company is betting heavily on four streams of revenue. The first is its small molecule active pharmaceutical ingredients (API) business, which supplies drug ingredients to other companies.
In the first quarter of 2015, Biocon filed a set of abbreviated new drug applications (ANDA) with the US Food and Drug Administration (FDA) requesting approvals to market new formulations of existing drugs. Unlike other Indian pharama companies such as Ranbaxy or Dr Reddy’s Laboratories, Biocon had not been selling small molecule generics in highly regulated markets like the US, but is now entering the market. It is putting in place a pipeline of difficult-to-make, technology-intensive molecules to tap the lucrative and growing international generic market.
Its second stream of revenue is its biosimilar drug business which, unlike small molecule generics, has exacting regulatory, clinical and development requirements because of which they are 20 to 50 times more expensive than conventional drugs. Biocon expects its biosimilar drugs to generate 20 percent of its sales by 2018, up from about 5 percent of revenues in FY12.
The third revenue stream includes branded formulations such as Insugen, Erypro Safe (nephrology), Statix (anti-cholesterol) and Telmisat (anti-hypertension), which are sold only in India. The pharma company’s presence in the chronic disease segment in the country is represented by over 80 brands spread across seven therapeutic segments: Diabetes, oncotherapy, nephrology, cardiology, immunotherapy, comprehensive care and bio-products.
And finally, there’s its decade-old novel molecule business. The Bangalore-headquartered company is working on six molecules (three for cancer, and one each for diabetes, auto-immune diseases and ophthalmology).
A pharma expert from a global consultancy says that Biocon has an edge over competition because of its collaborations with international behemoths such as Mylan and Advaxis. “These are big names in the pharma sector. The collaborations work in Biocon’s favour giving it a global edge and credibility,” he says.
Investing in Cancer
While diabetes has always been Biocon’s cash cow, in recent years Mazumdar-Shaw has also been focusing on cancer, which has emerged as a multi-billion dollar business in recent decades. “It is an immune-triggered disease. In a way, all of us live with cancer every day, but our immune system fights off these cells and that’s why we don’t get inflicted with the disease. But when our immune system for some reason cannot deal with these cancers, they fester. The cancerous cells grow and our body doesn’t know how to fight them,” she says.
In 2006, Biocon emerged as a serious player in this arena with the unveiling of its novel molecule Biomab-EGFR, India’s first indigenously developed human monoclonal antibody, which empowers a patient’s immune system to detect and kill head-and-neck cancers. (Simply put, monoclonal antibodies or mAbs are artificial versions of natural antibodies and are produced from a single clone of cells. These cloned antibodies hinder a cancer’s growth at the cellular level.)
Biocon is developing at least two biosimilar mAbs for oncology: Bevacizumab (for colo-rectal, lung, kidney and ovarian cancer, among others) and trastuzumab (breast cancer).
In February this year, it launched the breast cancer drug CANMAb (trastuzumab commercialised) in collaboration with US-based generic and specialty pharmaceutical company Mylan for the Indian market. The two companies are now developing trastuzumab for other markets.
CANMAb, which claims to be a biosimilar version of Roche’s Herceptin (a monoclonal antibody), fights the aggressive Her2-positive breast cancer. This type of cancer grows when a gene—which makes a protein called human epidermal growth factor receptor 2 (or Her2)—malfunctions, causing breast cells to grow and divide in an uncontrolled manner.
According to a report by the Mayo Clinic in the US, in one out of every five breast cancer cases, the cancer cells make an excess of Her2 due to a gene mutation. Biocon estimates that more than 1.45 lakh women in India are diagnosed with breast cancer every year, of which nearly a quarter test Her2-positive.
The sale of CANMAb will add to the company’s top-line, but Roche has filed for an injunction in a court in India to prevent Biocon and Mylan from comparing their biosimilar version to its Herceptin. (A Biocon spokesperson declined to comment on this saying the matter was sub judice.)
Treating Her2-positive cancer with Herceptin costs Rs 75,000 to Rs 80,000 for a 440 mg dose. CANMAb’s 440 mg dose sells at Rs 57,500. There’s also a 150 mg dose priced at Rs 19,500.
Every business unit at Biocon is immersed in cancer research. For instance, the company’s novel molecule arm is developing BVx 20—an anti-CD20 molecule for the treatment of non-Hodgkin’s lymphoma—with Vaccinex, a clinical-stage US biotech company. It is currently undergoing phase-1 clinical trials in India. In this phase, researchers test the drug on a small group of patients (no more than 80) for the first time to determine dosage and side-effects, and evaluate safety. (Clinical trials are allowed in India on a case-by-case basis and only after receiving approval from various government bodies.)
Biocon is also developing a novel cancer immunotherapy ADXS-HPV for treating cancers associated with the human apillomavirus. These include cervical, penile, anal and oropharyngeal cancers at the back of the throat. It has collaborated with Advaxis Inc, a clinical-stage biotechnology company, to develop and commercialise ADXS-HPV in India and other emerging markets.
Not only is Mazumdar-Shaw building an efficient drug production line, she is also trying to establish Biocon as a “cancer diagnostics and treatment” company. With good reason, too. She started the Mazumdar-Shaw Cancer Centre (MSCC) in Bangalore in 2009 and unveiled the non-profit research institute Mazumdar-Shaw Centre for Translational Research (MSCTR) this year. The 1,400-bed MSCC functions as a for-profit business for those who can afford treatment patients from low-income families get subsidised or free care.
“We need to understand cancer at the immune level,” says Mazumdar-Shaw, who has partnered with Bangalore-based silico-tech company Strand Life Sciences to develop low-cost genetic markers that can predict whether a person will develop cancer, as well as identify the gene responsible for it. “The faster we catch a cancer, the more likely is the success in treating it,” she says.
The World Health Organization estimates that nearly 5 lakh people die of cancer in India every year, and predicts that the number will rise to 7 lakh by next year.
 Banking on diabetes
Banking on diabetes
The incidence of diabetes is as alarming as that of cancer: According to data from the Indian Council of Medical Research (ICMR), India already has approximately 65.1 million diabetics, second only to China which has 98.4 million cases. In this field, Biocon is an old and experienced hand.
Diabetes and insulin production continue to be an integral part of Biocon’s agenda, and expansion is on the cards. Under its novel molecules active development programme, it has collaborated with American pharma company Bristol-Myers Squibb (BMS), to work on the oral insulin molecule, IN-105. Initial findings from the first set of trials in the US are expected at the end of this financial year. According to the US government trial monitoring website ClinicalTrials.gov, the study is still in phase-1. The purpose is to see whether the oral insulin is able to control increase in blood glucose after a meal. It will also tell whether a single tablet is safe for patients with type-1 diabetes.
If the initial readout is positive, Biocon will be a step closer to becoming the first company in the world to deliver insulin that can be taken orally instead of an injection. “This insulin tablet is coming along well,” is all Mazumdar-Shaw will say, for now.
Biocon had conducted a phase-III trial in India in 2010, which confirmed that the drug reduces blood glucose levels during and after meals, but it did not meet its primary endpoint. In the co-development partnership with BMS, Biocon aims to leverage the positive data obtained from the Indian phase-III study to design future studies that will target the right patient population. These are the trials that are ongoing in the US.
Apart from the insulin tablet, Biocon is working on eight biosimilar products under an exclusive arrangement with Mylan. This does not include the biosimilar drug, rh-insulin, which is being sold in India under the brand name Insugen. It’s planning to develop the drug for the European Union and US markets, and manufacture it at its Malaysian facility.
With Biocon’s production of insulin on a roll, the Malaysia facility will augment its existing capacities for insulin. It will initially produce rh-insulin and Glargine (a biosimilar insulin) at the plant. With an investment of around $200 million, the facility will be (after it receives the due clearances from the Malaysian regulators) the largest insulin-production plant in Asia.
Trying times
A sizable chunk of Biocon’s success hinges on its trials, and if it wants to conduct them in India, it has to adhere to national regulations.
Mazumdar-Shaw is frustrated by the stringent regulations governing clinical trials in India. “I believe this is an area that needs urgent attention because if the government continuously keeps denying companies the opportunity to clinically evaluate any kind of programme, we are not only devaluing these opportunities but also devaluing medical science in a big way,” she says.
This curb on trials is delaying Biocon’s pace of drug development, she points out. “It will increase R&D cost. The cost of doing trials in India to the cost of doing trials in the US is [higher by] a factor of 3 or 4, it is that much more expensive.”
That said, Mazumdar-Shaw is mitigating costs by bringing down the timeline. Citing an example, she says, in India, she had to wait for nearly a year to get approvals for trials of the oral insulin despite clearly demonstrating the efficacy and safety of the drug. “In the US, we started the new round of trials in 30 days,” she says.
The government regulations have negatively impacted the firm’s outsourced research business. Biocon offers integrated and value-added services that range from early discovery to late-stage clinical development through Syngene and Clinigene. “Our clinical services platform Clinigene is facing challenges because of the current Indian clinical trials environment. But it is not very significant to the company’s overall business. Further, Syngene, the discovery and development services platform, is seeing continued momentum that is more than compensating for the revenue loss,” says a company spokesperson.
An expert in the pharmaceutical industry who tracks Biocon expresses his faith in the company’s ability to deliver: “In this sector, the time required for trials in beyond one’s control, so we cannot hold that against Biocon or any other company. If we look at what they have right now, the business seems to be in a steady state.”
Silencing the critics
Market sentiment is on Biocon’s side. In the first week of September, analysts gave the company a ‘buy’ rating. Ashwani Gujral who heads an investment management firm gave it a ‘buy’ call with a target of Rs 525 and a stop loss of Rs 490 for the company’s stock. In July, an Edelweiss report had initiated coverage on the stock with a ‘buy’ rating and a target price of Rs 590 per share. Biocon was trading at Rs 491.85 on BSE as of September 11.
“We believe that the impending opening up of the biosimilar market (especially in the US), around a $71-billion market of biologics going off-patent, and high entry barriers for new players places Biocon at the forefront to monetise the lucrative opportunity and support growth over the long run,” analysts Vrijesh Kasera and Raksha Thadani wrote in their report for Edelweiss.
There are, however, some analysts who believe that lack of certainty for the timeline of the launch of the oral insulin and flat revenues in the biopharma segment for the past five quarters are areas of concern.
Shetty of HDFC Securities highlights some worrying trends including short-term capacity constraints because of the Malaysian plant.
“The demand is high and to cater to it, Biocon is setting up its Malaysian facility. But for the time being it means a lot of capital is being directed there,” she says.
This, however, is a temporary situation. In their report, Kasera and Thadani wrote: “We would further like to highlight that major upside in earnings is expected to kick in once the company gets regulatory clearances for the Malaysian facility by FY17.”
Biocon is also hostage to the political turmoil, conflict and lack of stable regimes in the Middle East and North Africa, especially because it exports many of its brands and formulations to countries in the region. Its first quarter results for FY2015 reflect this uncertainty. According to a recent report by global risk analytics company Maplecroft, “the combined intensifying political violence and resource nationalism in East Africa, is driving a global rise in political risks for investors.”
These are factors that are affecting almost all pharma companies and for Mazumdar-Shaw it’s just another hurdle to be crossed.
But she is all about facing the future. In April, Biocon appointed its COO Dr Arun Chandavarkar as chief executive officer and joint managing director. He was also inducted on to the board of Biocon. “We have already taken the first step towards it [succession]. Five to 10 years down the line I will be playing a more strategic role. We are grooming the team for the next level,” she says.
Once upon a time, Biocon was a startup, in a world where the phrase ‘startup ecosystem’ was an alien concept. This is something that Mazumdar-Shaw never forgets and it is reflected in her appreciation for the new generation of promising businesses.
On the morning that Forbes India met her in Bangalore, she had ordered a vacuum-cleaner for her mother. “My mother was planning to go to a store to buy the appliance. But John [her husband] and I told her that we could get it without the need to step out of the house.” While ordering a vacuum cleaner from Flipkart, she felt a kinship with the promoters of the Indian ecommerce website. “They [Sachin and Binny Bansal] had a vision, a passion and they worked towards it. Flipkart has revolutionised retail in the country,” she says.
Few can doubt that she has been a revolutionary of sorts as well.
First Published: Sep 30, 2014, 06:33
Subscribe Now